Patient Submission Process Overview
The National Centre for Pharmacoeconomics is committed to facilitating the involvement of patients in the Health Technology Assessment (HTA) process. We believe that patients have perspectives and experiences that can uniquely contribute to the decision making process. With this in mind, the NCPE provide the Patient Organisation Submission process, to enable patient groups to communicate their experiences directly to the decision maker, the Health Services Executive (HSE).
The Patient Organisation Submission Process encourages Patient Organisations to gather information from their members for inclusion in the Patient Organisation Submission of Evidence Template. In particular, this template includes information on the day-to-day experience of living with the disease and the ways in which the new drug may improve this day-to-day experience. This information can help the HSE Drugs Committee to understand the real-world impact a new drug may have on the quality of life and daily experience of patients and carers. The HSE Drugs Committee considers the findings of the NCPE HTA report in line with other criteria as defined in the Health Act 2013
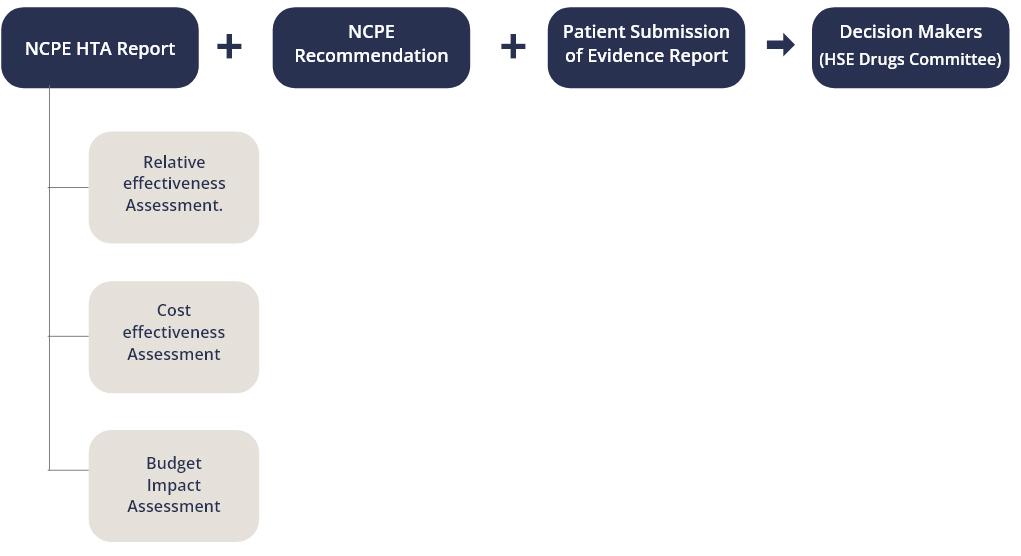
Process Overview
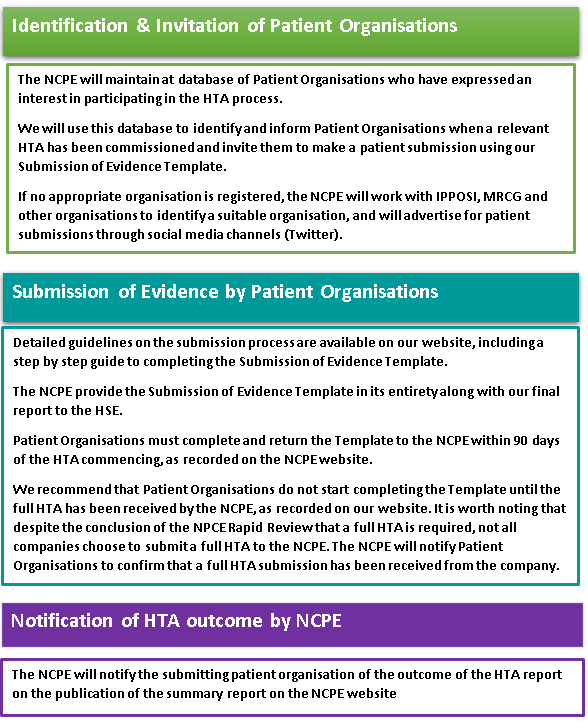
The NCPE have identified three key steps in the Patient Submission Process; Identification and Notification of the Patient Organisation, Submission of the Patient Organisation Submission of Evidence Template, and Notification of Outcome by the NCPE.
- Identification & Invitation
- Submission of Evidence
- Notification of Outcome
The NCPE will maintain at database of Patient Organisations who have expressed an interest in participating in the HTA process.
We will use this database to identify and inform Patient Organisations when a relevant HTA has been commissioned and invite them to make a patient submission using our Submission of Evidence Template.
If no appropriate organisation is registered, the NCPE will work with IPPOSI, MRCG and other organisations to identify a suitable organisation, and will advertise for patient submissions through social media channels (Twitter).
Detailed guidelines on the submission process are available on our website, includinga step by step guide to completing the Submission of Evidence Template.
The NCPE provide the Submission of Evidence Template in its entirety along with our final report to the HSE.
Patient Organisations must complete and return the Template to the NCPE within 90 days of the HTA commencing, as recorded on the NCPE website.
We recommend that Patient Organisations do not start completing the Template until the full HTA has been received by the NCPE, as recorded on our website. It is worth noting that despite the conclusion of the NPCE Rapid Review that a full HTA is required, not all companies choose to submit a full HTA to the NCPE. The NCPE will notify Patient Organisations to confirm that a full HTA submission has been received from the company.
The NCPE will notify the submitting patient organisation of the outcome of the HTA report on the publication of the summary report on the NCPE website